Energy exists in many different forms. The different
types of energy include light energy, heat energy, chemical energy, sound energy,
etc.
Each form can be converted or changed into other
forms.
For example, when you burn a piece of wood, the
chemical energy stored in the wood changes to heat and light energy.
The branch of science which deals with the study of different forms of
energy and the relationships between them is known as Thermodynamics.
TERMS USED IN THERMODYNAMICS:
SYSTEM AND SURROUNDINGS
When a
reaction occurs in a jar, everything inside the jar is the system and
everything outside the jar is the surroundings.
The part of
the universe which is kept under observation is known as the System and the
remaining portion of the universe is called Surroundings.
WORK
Work is said
to be done when an object moves as a result of force (push or pull) applied to
it.
It is the
product of force and distance (or displacement).
Work done=
Force X Displacement
W= F X dl
The S.I. unit
of work is Joules.
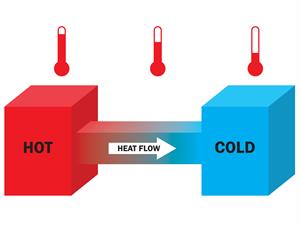
HEAT
It is a form
of energy that can be exchanged between the system and the surroundings due to
temperature difference (flowing from high temperature body to low temperature
body).
The S.I. unit
of heat is also Joules.
STATE OF A SYSTEM
The state of
a system means the condition of the system which is described in terms of
observable properties like pressure, volume, temperature, etc.
“Con entusiasmo, todo es posible.”
“With
enthusiasm, everything is possible.”

Comments
Post a Comment