An atom is the smallest particle of an element that can take part in
a chemical reaction. It may or may not exist independently.
A molecule is the smallest particle of an element or a compound that
can normally exist independently.
A substance that cannot be decomposed into simpler substances by chemical means and is made up of only one kind of atoms is called an element. The smallest particle of an element is an atom.
A substance formed by the chemical combination of two
or more elements in fixed proportions is called a compound. The smallest
particle of a compound is a molecule.
When two or more elements combine together not
chemically but physically we get a mixture.
Q. Why are the properties of different compounds so different from each other?
A. The properties of different
compounds are different from each other because the molecules of the compounds
are different from each other.
Q. Why are most elements not found in
the free state in nature?
A. Many elements have a great tendency
to combine with each other to form compounds. These elements are, therefore,
not found in the free state in nature.
Properties of compounds:
1) A compound can be broken down into its
constituent elements by chemical methods.
2) A compound always contains the same
elements combined together chemically in a fixed ratio.
3) The properties of a compound are
different from those of its constituent elements.
Difference between compounds and
mixtures:
1) In compounds, constituents are
combined together chemically in a fixed ratio whereas in mixtures, constituents
are combined physically in any ratio.
2) In compounds, constituents can be
separated by chemical methods whereas in mixtures, constituents can be
separated by physical methods.
3) Energy is absorbed or released
during the formation of compounds whereas no energy is absorbed or released
when a mixture is formed.
Writing the full names of elements and
compounds, and describing their reactions is very inconvenient. It is more
convenient to use abbreviations or symbols. We use symbols to represent to
elements. A compound is represented by a formula which contains symbols of all
the elements present in a molecule of that compound.
Some common elements and their symbols:
Element
Symbol
Aluminum
Al
Calcium
Ca
Carbon
C
Chlorine
Cl
Copper
Cu
Gold
Au
Helium
He
Hydrogen
H
Iron
Fe
Nitrogen
N
Oxygen
O
Potassium
K
Sodium
Na
Sulphur
S
Tungsten
W
Zinc
Zn
Atoms of some elements cannot exist
independently in nature. They form molecules containing two or more atoms.
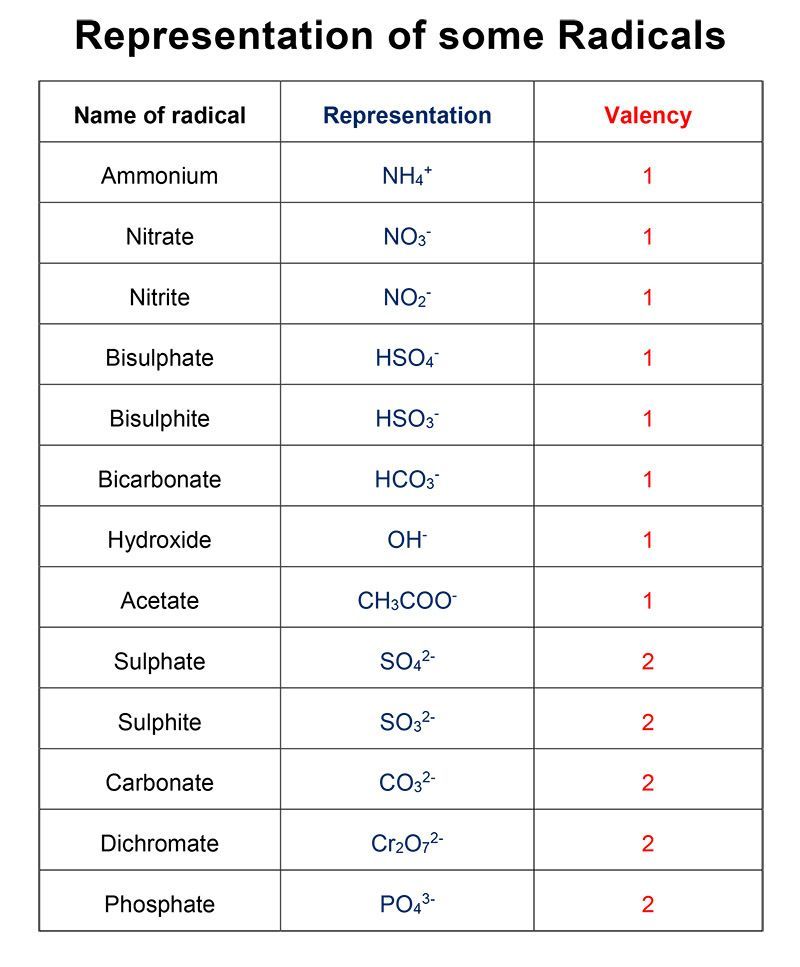
A group of atoms carrying a positive or negative charge is known as radical. Examples are ammonium and sulphate.
The number of atoms present in a molecule of an element is known as its atomicity. If a molecule consists one atom it is known as monoatomic element. If a molecule consists two atoms it is known as diatomic element.
What information does a formula give?
1) It tells the type of elements present in the
compound.
2) It tells how many atoms of each element are present
in the compound.
3) The formula represents the molecule of the
compound.
Chemical formula of ammonia is
There are two elements present in ammonia- Nitrogen
and Hydrogen. A molecule of ammonia contains one atom of nitrogen and three
atoms of hydrogen. It represents one molecule of ammonia.
The valency of an element denotes its
combining capacity. We take the valency of hydrogen as 1. The valencies of
other elements is the number of hydrogen atoms which can combine with one atom
of that element. Example In ammonia one atom of nitrogen combines with three
atoms of hydrogen, the valency of Nitrogen will be 3.
Writing a formula
1) Write the symbols of the elements side by side.
Write their valencies below the symbols.
2) Divide by any common factor in their valencies.
3) Interchange the numbers obtained and write them at
the base to get the formula.
The elements that take part in a chemical reaction are
known as Reactants. The new compounds
formed in the chemical reaction are called Products.
A chemical
equation shows the result of a chemical reaction in which
the reactants and the products are represented by symbols or formulae.
A balanced chemical
equation is the one in which the number of atoms of each
element on the left hand side are same as the number of atoms on the right-hand
side.
This is an example of a balanced chemical equation. In the above
reaction, nitrogen and hydrogen are the reactants (written on the left hand
side) and ammonia is the product formed (written on the right hand side).





Comments
Post a Comment