Electrons are negatively charged particles
whereas protons are positively charged particles.
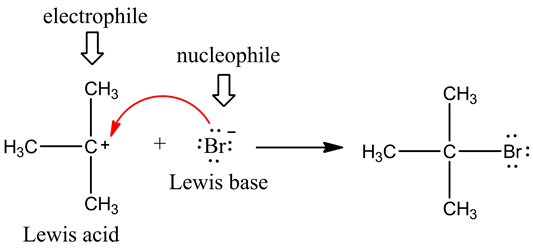
Electrophiles are electron loving chemical species.
Electrophiles may be either positively charged or
neutral species.
Electrophile
examples: H⊕, Cl⊕, Br⊕, CH3⊕, SO3, BF3
Electrophiles have a lack of electrons so they are
capable of accepting or taking electrons from electron rich species.
Electrophiles accept electrons and hence behave
as Lewis acids.
Nucleophiles are nucleus loving chemical species.
Nucleophiles may be either negatively charged or
neutral species.
Nucleophile examples: Cl⊝, Br⊝, CH3⊝, OH⊝, NH2⊝, H2O, NH
Nucleophiles have an excess of electrons so they
are capable of donating or giving electrons to electron deficient species.
Nucleophiles
donate electrons and hence behave as Lewis bases.
Electrophile taking electrons from nucleophile :P
“Milagros pueden suceder.”
“Miracles can happen.”

Comments
Post a Comment