Entropy is a
measure of disorder or randomness of a system.
The term
order means presence of symmetry whereas disorder means lack of symmetry.
Entropy (or disorder)
is all around you.
Cells within
your body are dying, the floor is getting dusty, conflicts are taking place
around the world, cars are prone to rusting, etc. The entire universe is
marching towards chaos or disorder. This suggests that the entropy (or
disorder) of the universe is always increasing.
Energy or work must be put into the system, in order to bring the system back to a state of order because a state of order leads to stability whereas disorder leads to instability or chaos. For example, cars are prone to rusting, therefore, a car must be maintained with work in order to keep it in an ordered state to prevent rusting.
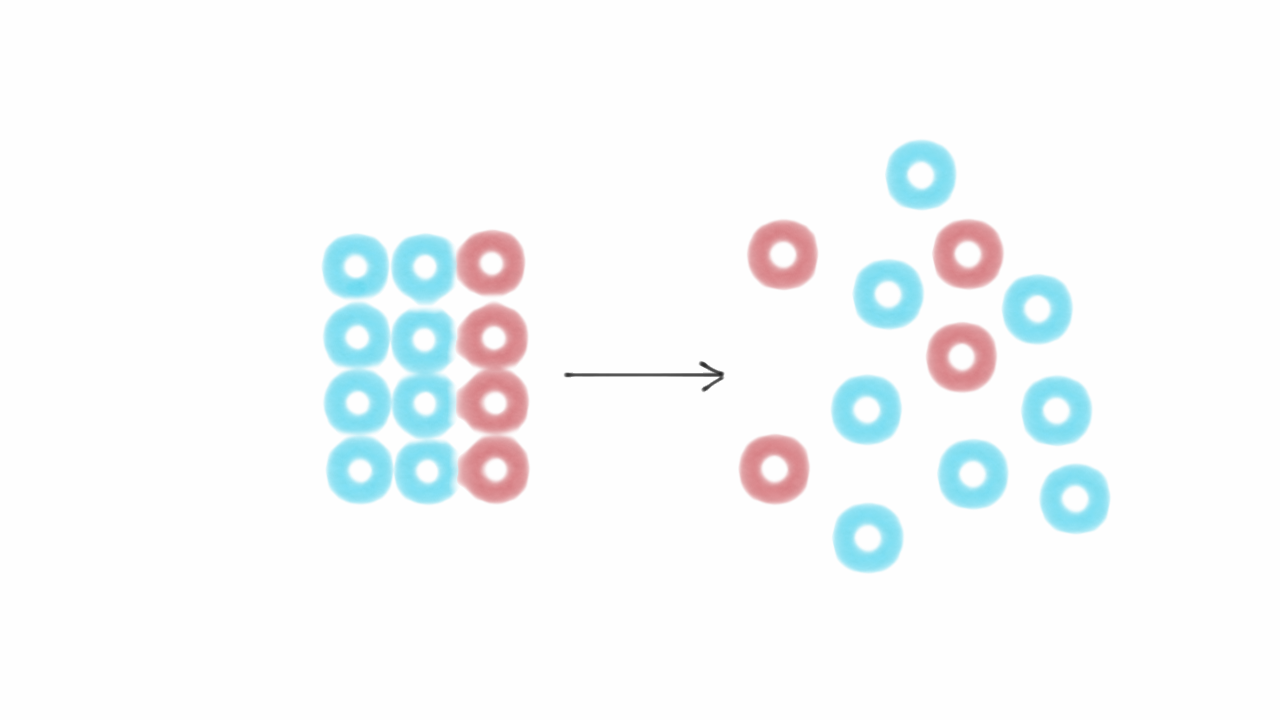
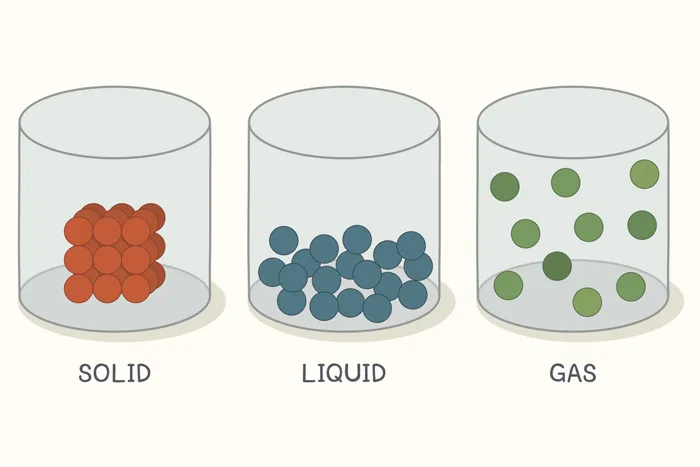
The greater the disorder in a system, the higher is the entropy.
For a given substance, the solid state is the state of lowest entropy
(most ordered state) because the particles of solids have ordered arrangement
with little movement.
“La felicidad es la más alta forma de salud.”
“Happiness is the highest form of health.”
Comments
Post a Comment